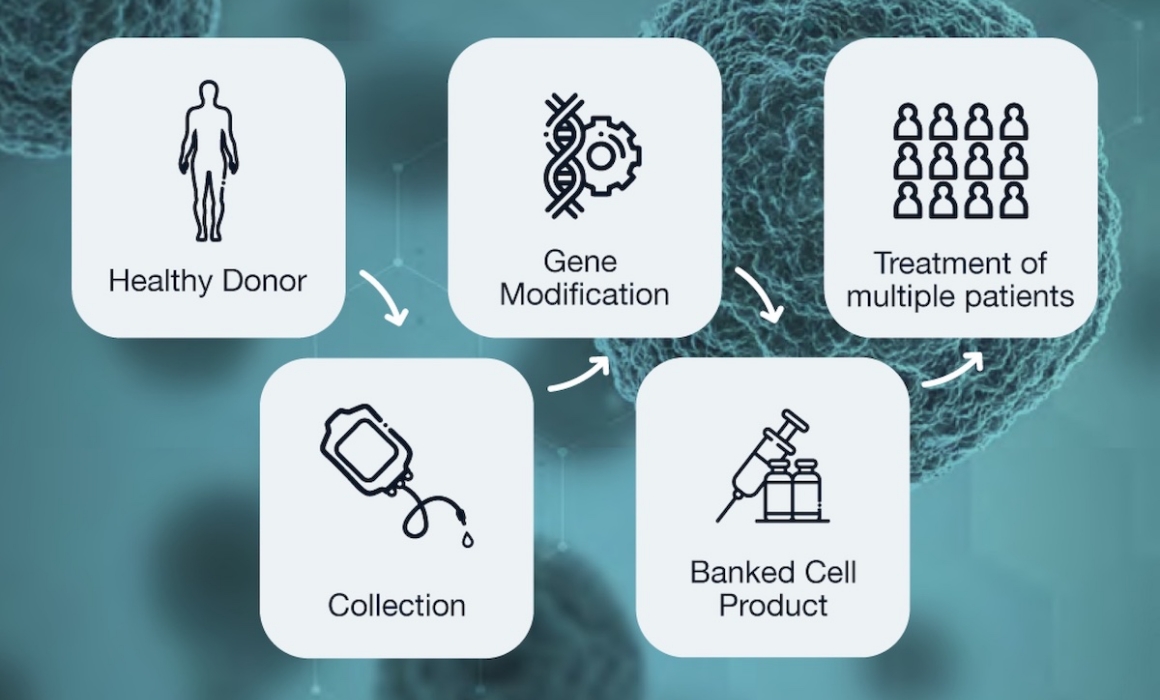
Center for Breakthrough Medicines (CBM) is a CDMO dedicated to bringing capabilities to the full cell therapy manufacturing process from early development to large-scale commercial production. We can advance your cell therapy product to IND through commercialization, and our clients can leverage a single-sight CDMO facility, capable of fully integrated production, with flexible, forward-engineered suites and client-centric program management.
Cell Therapy Manufacturing CDMO Services
Autologous and Allogeneic CDMO Capabilities
Starting Material Plasmid & Vector
- • Off-the-shelf or custom manufacturing of expression and packaging plasmids with clear title and non-restricted IP
- • Lentivirus manufacturing for gene-modification
Process & Analytical Development
- • CBM can either develop and optimize client-specific processes or tech transfer in established processes seamlessly transfer your manufacturing process
- • Utilize the same equipment and scale during development and tech transfer as in your GMP facilities
- • Scale up with pilot and training runs in one location to advance your project to cGMP production
- • Draft electronic batch records in pilot runs for efficient debugging to de-risks GMP manufacturing
- • Experienced program management team giving you clear oversight of your project through regular updates, on-site access, and the potential for dedicated office space onsite.
GMP Cell Processing
- • For autologous cell therapy products, we employ sophisticated scheduling, supply chain and inventory control systems aligned with in-process testing, quality control, and lot release programs to overcome challenges. A structured process ensures integrity through Chain of Identity and Chain of Custody from receipt of donor/patient material to processing, storage, and shipment back to the Authorized Treatment Centers.
- • For allogeneic cell therapy products, well-characterized cell banks may be scaled up with suspension systems using single-use bioreactors to meet clinical and commercial demand for the cell products.
- • Cell-derived therapies include CAR-T, TCR-T, Dendritic, Natural Killer, TIL, Treg, monocytes, and macrophages
- • Tissue-derived therapies can be started from TIL, induced pluripotent, mesenchymal stem cells, hematopoietic stem cells, and other regenerative therapies
Testing & Quality Control
- • World-class Manufacturing Execution and Quality systems minimize manufacturing hiccups while streamlining efficiency enabling parallel batch processing and reducing manual data entry and review times by 50-70%. This translates quality control (QC) team operating in direct conjunction with manufacturing for method transfer, optimization, qualification, and validation. Testing and analytical labs are directly adjacent to production for rapid and seamless cGMP testing.
- • Core cell therapy analytical methods include:
- qPCR, rapid micro methods, flow cytometry, ELISA, and automated cell counting.
- Incoming raw material, in-process and final product testing.
- Network of qualified contract testing laboratories to supplement in-house capabilities.
- Environmental, personnel and utility monitoring.
- Starting Material Plasmid & Vector
-
Starting Material Plasmid & Vector
- • Off-the-shelf or custom manufacturing of expression and packaging plasmids with clear title and non-restricted IP
- • Lentivirus manufacturing for gene-modification
- Process & Analytical Development
-
Process & Analytical Development
- • CBM can either develop and optimize client-specific processes or tech transfer in established processes seamlessly transfer your manufacturing process
- • Utilize the same equipment and scale during development and tech transfer as in your GMP facilities
- • Scale up with pilot and training runs in one location to advance your project to cGMP production
- • Draft electronic batch records in pilot runs for efficient debugging to de-risks GMP manufacturing
- • Experienced program management team giving you clear oversight of your project through regular updates, on-site access, and the potential for dedicated office space onsite.
- GMP Cell Processing
-
GMP Cell Processing
- • For autologous cell therapy products, we employ sophisticated scheduling, supply chain and inventory control systems aligned with in-process testing, quality control, and lot release programs to overcome challenges. A structured process ensures integrity through Chain of Identity and Chain of Custody from receipt of donor/patient material to processing, storage, and shipment back to the Authorized Treatment Centers.
- • For allogeneic cell therapy products, well-characterized cell banks may be scaled up with suspension systems using single-use bioreactors to meet clinical and commercial demand for the cell products.
- • Cell-derived therapies include CAR-T, TCR-T, Dendritic, Natural Killer, TIL, Treg, monocytes, and macrophages
- • Tissue-derived therapies can be started from TIL, induced pluripotent, mesenchymal stem cells, hematopoietic stem cells, and other regenerative therapies
- Testing & Quality Control
-
Testing & Quality Control
- • World-class Manufacturing Execution and Quality systems minimize manufacturing hiccups while streamlining efficiency enabling parallel batch processing and reducing manual data entry and review times by 50-70%. This translates quality control (QC) team operating in direct conjunction with manufacturing for method transfer, optimization, qualification, and validation. Testing and analytical labs are directly adjacent to production for rapid and seamless cGMP testing.
- • Core cell therapy analytical methods include:
- qPCR, rapid micro methods, flow cytometry, ELISA, and automated cell counting.
- Incoming raw material, in-process and final product testing.
- Network of qualified contract testing laboratories to supplement in-house capabilities.
- Environmental, personnel and utility monitoring.
CBM Thought Leadership

Webinar: Scaling Cell Therapies: Auto vs. Allo
To achieve commercial success, a developed process must be scalable and suitable for a manufacturing environment. The scaling strategy depends on the type of cell therapy. A deep dive into why collaboration between process development and manufacturing leads to accelerated timelines, lowered costs, and improved risk assessment for both autologous and allogeneic cell therapies.

CBM Expertise
Our capabilities are co-located on a single campus for seamless hand-off between manufacturing and testing to reduce vein-to-vein time of autologous cell therapies for the patients who need it the most. We offer unparalleled flexibility to work alongside our subject matter experts in development labs and during GMP operations in our manufacturing suites.
Looking for support for your upcoming cell therapy manufacturing program?