Accelerating AAV Analytical Development And Testing With Advanced Methods And Comprehensive Solutions

Advances made in the gene therapy space over the last several years have underscored the importance of accelerated innovation for its manufacturing. Adeno-associated virus (AAV) gene therapies represent a promising modality for the space, owing to a number of preclinical and clinical successes in the last few years related to AAV-mediated gene replacement, silencing, and editing. This work has resulted in the commercialization of four AAV gene therapies in the U.S. and EU – the spinal muscular atrophy treatment Zolgensma; Luxturna, which targets inherited retinal diseases; Upstaza, used to treat children with aromatic l-amino acid decarboxylase (AADC) deficiency; and the hemophilia drug Roctavian.
These drugs represent life-changing intervention for patients. As more AAV gene therapies progress through the pipeline into clinical trials, enabling their successful development and commercialization will be critical to bringing more cures to patients. Testing drug product is a core component of that enablement and while many strides have been made in bringing additional capacity online for the manufacturing of AAV gene therapies, a persistent lack of comprehensive, optimized testing capabilities has created a landscape where testing backlogs, lengthy method transfer timelines, and large sample volumes needed are the norm. Finding a contract development and manufacturing organization (CDMO) that can perform all the necessary testing for an AAV product, while preserving precious drug product and adhering to or accelerating timelines, can make a significant difference for a nascent company or novel drug.
The Challenges of Contract Testing: Wait Times, Delays, and Lack of Expertise
There are a number of factors that have served to create these challenges, from uneven or nonexistent testing capacity for many CDMOs to a lack of expertise or access to appropriate or optimal assays.
In a recent survey conducted by Deloitte, CMC and quality testing decision-makers noted that:
– 86% of testing providers lacked a specific assay or technique
– 57% had wait times of 12+ months for initiating tests
– 71% took too long to develop custom method
Additionally, the technical challenges associated with AAV manufacturing (such as managing risk from the PEI, adventitious agents, resins, and detergents) require companion analytics capable of effectively evaluating their toxicity and safety profile during development and through commercialization. Finally, issues related to yield for AAV products require testing solutions that are able to accommodate the assays necessary for regulatory compliance while using as little drug product as possible.
Another major hurdle to achieving faster, more streamlined analytical testing relates to method transfer – for those outsourcing to multiple third parties, the burden of transferring analytical methods takes on additional complexity. With variability from lab to lab and instrument to instrument along with the knowledge gaps created by siloed testing can lead to backlogs and disconnect that resulting in cascading delays.
As a result of this lack of comprehensive testing capacity, many organizations have been forced to construct a complex network of outsourced vendors to perform AAV testing. Even biotechs contracting with CDMOs may be subject to a process wherein the majority of the analytical assays necessary to their GMP manufacturing are performed using multiple third parties (up to 5+ according to survey respondents in Outsourced Pharma’s recent CGT Capacity Update). Advanced analytical platforms utilizing more sensitive methodologies can help operators achieve a testing paradigm that conserves valuable drug product by minimizing sample volume requirements. This, coupled with instruments that demonstrate greater throughput, robustness, precision, and sensitivity, can serve to minimize the potential for testing rework, further optimizing a process and accelerating timelines.
As an example, mass photometry can be used instead of analytical ultracentrifugation to evaluate empty and full viral particles and aggregation. Mass photometry is an advanced analytical tool for evaluating viral particles and the extent of genomic content. Empty, full, and intermediate species containing incomplete vector genome can be measured with a high degree of resolution. Unlike traditional techniques, mass photometry measures the true molecular mass and is also more rapid, enabling faster time to data. Finally, mass photometry requires very little sample volume (up to 50% less), thus conserving precious AAV batch volume and drug product. Taken together, mass photometry is an exciting new technology that may help get novel drugs to market faster.
This focus on improving the specificity and sensitivity of testing instrumentation is crucial to establishing a paradigm that improves product release and minimizes loss; potency assays that distinguish between full, empty, and partial capsids, for example, can help operators improve specification, tighten a product’s safety profile, and optimize purity for improved drug product. In contrast, many CDMOs that have worked to establish testing expertise have only done so for one or two core assays and are unable or unwilling to adapt existing protocols to a specific drug. Because these drugs are so novel and often differ from product to product based on manufacturing approach, disease indication, or serotype, having the built-in flexibility to adapt a testing approach to a given product is crucial for the space.
A Better Approach to Contract Testing
Finding a manufacturing partner that provides comprehensive, optimized AAV testing can help innovator companies greatly improve the efficiency and consistency of their processes, accelerating timelines and reducing unusable or suboptimal testing. While some contract testers are delayed by instrument procurement and talent acquisition after initiating a program, SK pharmteco is built-to-spec, having invested heavily in equipment, instrumentation, and expertise centered on improved testing.
By working toward a comprehensive testing paradigm that convenes much of the necessary AAV assays (over 40+) and focusing on advanced analytical techniques that reduce sampling and improve speed, we are able to accelerate time to GMP batch release more than three-fold from 22 weeks to 6 weeks.
SK pharmteco has also worked to develop testing methods that are well aligned to process monitoring and quality control. Our tests align with the FDA domains for safety, potency, purity, identification, and stability, and the breadth of its testing offering has created compounding advantages for partners. SK pharmteco’s extensive menu and our ability to perform the bulk of the testing for an individual process provides team members with familiarity on specific applications and products such as AAVs. This enables faster, more responsive investigations in the event of invalid test results, as well as improved oversight of in-process testing. This is true regardless of whether SK pharmteco is a client’s AAV manufacturing partner or even if drug product comes from another source – such as internal manufacturing or another CDMO.
Saving Time, Costs, and Product with Optimized Testing
As one example of our commitment to optimizing testing, we provide our clients faster options to determining viral titer (Figure 1). Determining the physical titer of AAVs has typically been accomplished using digital droplet PCR (ddPCR) and SK pharmteco provides platform methods for AAV using the QX ONE and QX200 systems. That said, droplet-based methods, however, can be challenged by matrix effects and long analysis times (up to 7 hours). With advanced nanoplate-based digital PCR technology (dPCR), it is possible to achieve accurate titers (Figure 2) in 2-hour run times using simple workflows, even for challenging samples. The multiplexing capabilities of a digital PCR system allow quantification of more targets using fewer assays, accelerating time to market. Digital PCR can also be performed on a more diverse set of sample matrices, enabling titering at more stages of AAV production to ensure product quality, safety, and efficacy.
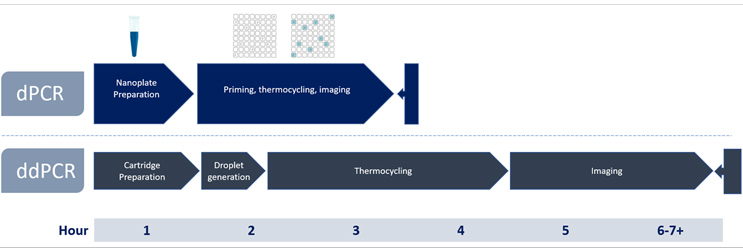 Figure 1: dPCR vs ddPCR Titering Workflow
Figure 1: dPCR vs ddPCR Titering Workflow
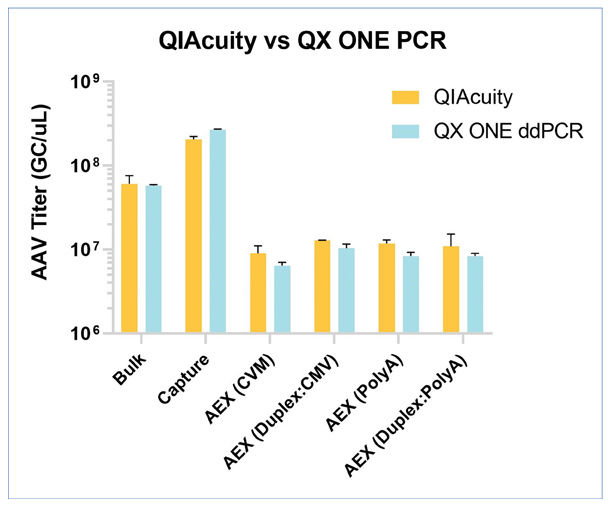
Figure 2: Comparison of results for different AAV8 samples obtained using dPCR ddPCR systems. The bulk and affinity results are for CMV-Cy5 singleplexes
Emphasis on analytical depth and breadth such as the above example has served to markedly decrease GMP testing turnaround time. For SK pharmteco, this has equaled a 70 percent improvement in turnaround time (an average of six weeks to completion, compared to 22 weeks for more typical paradigms). Additionally, SK pharmteco is able to cut custom method development time in half. Through close, collaborative client relationships, SK pharmteco is able to engage in accelerated method transfers and testing, engaging in frequent data sharing and troubleshooting. SK pharmteco has likewise prioritized client insight into testing – its LIMS system allows to track a product’s analytical testing. SK pharmteco is also developing apps and other tools to provide clients with greater transparency into their testing programs.
Conclusion
With more than 60 AAV trials currently underway in the U.S. alone, the future of these drugs rests on pioneering analytical approaches that are flexible, comprehensive, and right-sized for a therapy. As a full-service contract development, manufacturing, and testing organization, SK pharmteco can offer clients a full range of analytical assays for their AAV product, regardless of source or phase of clinical development. We’ve worked to greatly expand capacity, with more than 200,000 sq. ft and 30+ testing labs dedicated to advanced therapy product testing. This comprehensive testing platform, coupled with SK pharmteco’s in-house analytical expertise and collaborative data sharing, can help clients achieve faster, more streamlined development and a more efficacious, safer drug product.
