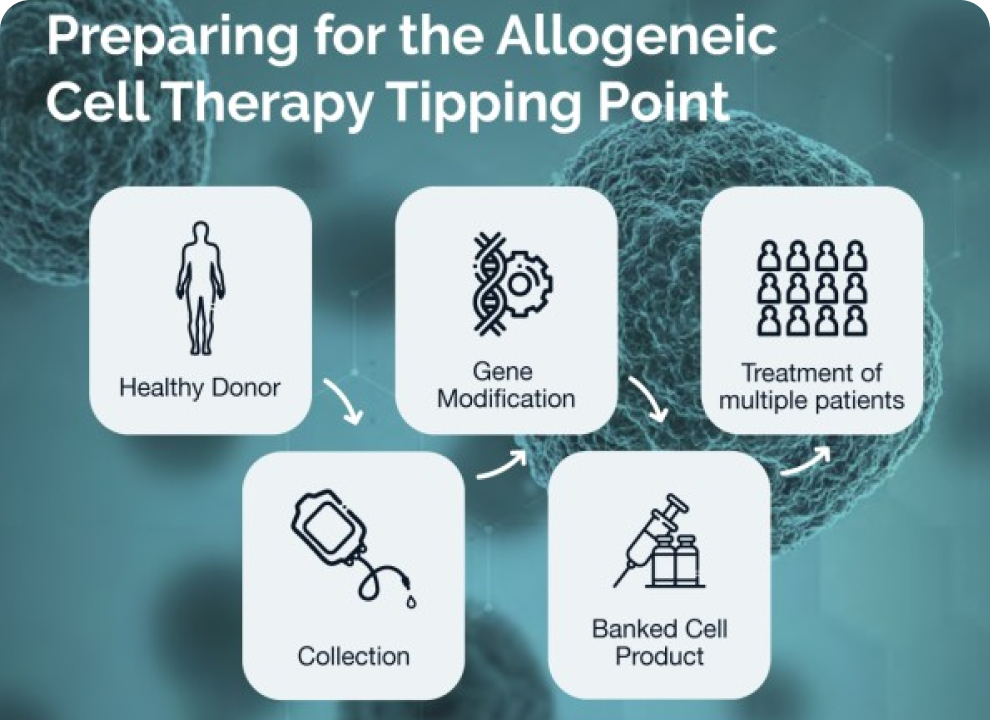
The Center for Breakthrough Medicines (CBM) is a single-source CDMO providing end-to-end solutions for the development, manufacturing, and commercialization of advanced therapies.
Our fully-integrated facility is positioned to support innovative cell and gene therapy programs at every stage of the development cycle to rapidly deliver lifesaving therapies to patients that need them.